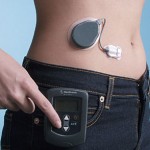
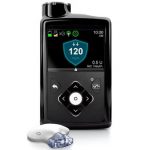
Adding a Second Medication to Artificial Pancreas Systems
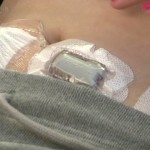
In people who do not have diabetes, the hormone amylin is secreted in the pancreas alongside insulin, and people with diabetes lack both insulin and amylin. Do artificial pancreas systems perform more effectively when pramlintide, a medication that is an analog of amylin, is added to them? Read more